|

|
|
IndiaRubberDirectory.com > Rubber Engineering > Elastromer Properties |
The Physics of
Rubber
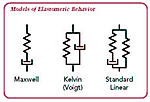
Rubber is composed of long chains of randomly
oriented molecules. These long chains are subject to entanglement and
cross-linking. The entanglement has a significant impact on the viscoelastic
properties such as stress relaxation. When a rubber is exposed to stress or
strain energy, internal rearrangements such as rotation and extension of the
polymer chains occur. These changes occur as a function of the energy applied
and the duration and rate of application, as well as the temperature at which
the energy is applied.
A rubber’s response to an applied energy can be
energy storage (elastic) or energy dissipation (viscous). For sealing elastomers,
the elastic component of response is most important. An applied stress induces a
corresponding strain which creates contact stress (or sealing force). As the
polymer chains rearrange to reduce this internal energy, or stored force, a loss
of sealing force occurs.
Rubber products are typically cured at high
temperature and pressure. The addition of curatives and accelerators forms
cross-links between the polymer chains or backbone. It is this network of
cross-links that largely determines the physical properties of tensile,
elongation and compression set.
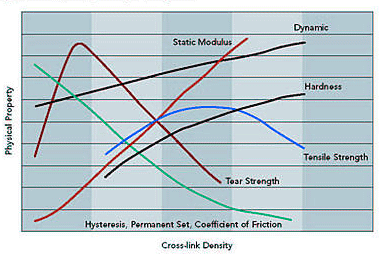
Fillers play a large role in rubber technology.
Carbon black and silica fillers can serve to improve the hardness, abrasion
resistance, tensile properties and tear strength. Non-black fillers, such as
titanium dioxide and barium sulfate can offer pigmenting properties for part
identification, as well as improved stability in strong oxidizing environments.
However, the viscoelastic response and hysteresis losses are greatly enhanced by
the use of fillers.
The physical properties of an elastomer vary with
the test conditions—especially temperature. The rate of application of a load
also has an effect, as does previous stress history.
|