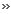Chloprene Rubber (CR) is the earliest commercially successful synthetic
rubber. This was introduced by Dupont under the trade name ‘Duprene’ in
1932. Later is was sold under the trade name ‘Neoprene’. During the
second World War, US Government designated this as GR-M (Government
Rubber - Mono Vinyl Acetylene). This is now being commercially produced
by several large companies including Dupont.
Chloprene rubber provides a very useful all-round balance of properties.
The monomeric unit of
CR is 2-chloro-1,3-butadiene.
CR is a sterio regular rubber, being in fact largely
trans - 1, 4 Poly Chloprene (88%). Cis accounts for 9.5% 1,2 around 1.5%
and 3,4 addition around 1%. The 1, 2 addition makes Chlorine available
in the principle site of vulcanization. The trans content attributes
crystalline
nature on stretching. The gum vulcanization of Chloroprene have high
tensile strength. It has moderate resistance to swelling in
oils/solvents combined with good resistance to low temperature
softening. It shows good resistance to weathering, heat ageing,
oxidative and ozone ageing.
Production of Chloroprene Rubber
CR is produced free-radical emulsion polymerisation
of chloroprene. Chloprene is produced from butadiene.
Two types of processess are usually used to emulsion polymerisation of
Chloroprene :
(1) Sulphur modified or Thiuram modified
The control of the polymer molecular weight is achieved by
copolymerisation. Polymer chains are cleaved at the sites where the
Sulphur is copolymerised.
(2) Mercaptan modified
The control of the molecular weight is achieved by the inclusion within
the polymerisation receipe of chain transfer agent.
When Chloprene is emulsion polymerised in the absence of any above
modifiers the product form even at low conversion is a tough, insoluble,
non-plastic material. Addition of 0.2 - 1.5% of Sulphur is dissolved
form in the monomer is still not suitable as an elastomer. If polymer in
the latex form is heated in an alkaline medium with a ‘thiophilic’
agent, such as Tetraethyl Thiuram Disulphide the polymer becomes
plasticised to a soft, soluble Polychloroprene which is suitable as an
elastomer.
Outline of Process
(1) Sulphur and Rosin-acid soap dissolved in Chloroprene monomer.
(2) Emulsification of solution in water in which NaOH and sodium salt of
the naphthalene sulphonic acid - formaldehyde condensation product have
been dissolved.
(3) Insitu formation of soap-sodium resinate at the interface between
hte Chloroprene drop lets and the aqueous phase. This results in the
formation of a stable emulsion of Chloroprene in water.
(4) Emulsification achieved by re-circulation the Chloroprene-Water
mixture through centrifugal pump.
(5) Polymerisation in an agitator at controlled temperature. Extend of
polymerisation measured by the specific gravity of reaction mixture.
(6) Polymerisation stopped by addition of Tetraethy Thiuram disulphide.
This acts as the stabiliser also.
(7) Acidification of alkaline latex to a pH of 5.5 - 5.8 by addition of
acetic acid. This also has the effect of arresting the petising action
of Thiuram disulphide, conversion of Sodium resinate back to Rosin acid
and also prepares the latex for the subsequent separation of the
polymer.
(8) Separation of polymer by continuous cogaulation of a polymer film,
followed by washing and drying.
Typical Polymerisation Receipe
Grading of Chloroprene Rubber
Chloroprenes are classified as general purpose or adhesive types. The
general purpose types are divided into three classes : G, W and T. G
types are copolymerised with sulpur and thiuram disulphide. The W types
are modified during manufacture with a mercaptan which gives them
greater storage stability and are more crystallization resistant. These
grades normally require organic accelerators to cure reasonably fast.
The T types are closely related to W types and contain a polymer gel
fraction to imporve behaviour. These types have very low nerve and
shrinkage. The G, W and T types are further classified into different
grades. The general purpose types are used in a variety of
applications-particularly molded and extruded goods, hose helts, wrie,
cable, heets and soles, coated fabrics, gaskets, etc.
Adhesive types
Different grades of adhesive types of Neoprene is available-Neoprenes
AC, AD and CG show a high degree of crystallinity. Neoprene AF shows a
low degree of crystallinity.
Toluene is usually used as a solvent for dissolving AC, AD or CG in
either chip or milled forms. With AF, some polar solvent should be used
with toluene to increase polymer solubility.
Compounding
In curing Neoprenes, metallic oxides are considered essential with 4
parts of light calcined MgO and 5 parts of ZnO standard. The W and T
grades do require organic acceleration and thiourea at 0.5-1.0 part is
probably the most economical. All Neoprene compounds require 1-2 Phr
antioxidant for good ageing resistance. For reinforcement blacks,
blacks, silica or hydrated calcium silicate may be used. Whiting is an
extender in neoprene. Petroleum oils are used as softeners in Neoprene,
usually aromatic. Processing aids include low molecular weight
Polyethylene, Microcrystalline waxes and palm oil. CI resins and
hydrogenated rosin esters are used as tackifiers.
When water resistance is required red lead (Pb3O4) is used as the curing
agent in place of MgO and ZnO.