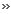NR is present to the
extent of about 94% in the non aqueous component of latex and the
other main items are carbohydrates, proteins, sterols, lipids,
carotenoids, etc. The non-rubber components present in latex have
pronounced
effect on the reactions
of NR E.g.: in the rate of vulcanization.
NR is structurally
almost completely Cis 1,4 Polyisoprene the monomer joined in head to
tail fashion. The polymer is also found to have chain branching
resulting from the presence of small numbers of non-hydrocanbon groups
distributed along the chains. Thus carbonyl, epoxy, lactone and amine
groups have been reported to be present in the hydrocarbon chain. The
phenomena known as ‘Storage hardening’ or NR studies have revealed the
presence of a sequence of 3 trans units/chain in hevea rubber
hydro-carbon. The likely structure of NR is given below.
Where X is H or COR, n
10000.
The presence of a
double bond in BR makes the molecule reactive; addition to the double
bond and substitution of the 7 active hydrogen atoms (one hydrogen
atom viz., Vinylic hydrogen is not reactive) are possible. The
presence of a - CH3 group in the monomer increases the electron
availability at the double at the double bond thereby increasing its
reactivity towards electrophilic reagents. The 3 alkyl groups help to
stabilise the resulting positive ion or free radical derived from the
olefin by hydrogen abstraction from the L - carbon atoms. Thus NR
undergoes addition reactions with Chlorine, Bromine, Iodine Chloride,
Hydrogen halides, Hypochlorus Acid, etc. Epoxidation of NR using
reagents like Hydrogen peroxide and acetic acid has evinced
considerable interest recently since epoxidised NR has been found to
possess properties like oil resistance, lower air permeability than
NR, etc. Presence of double bond also makes the NR susceptible to
chain degradation. Thus during the processing of NR in a consumer’s
factory, it is broken down in a mixing mill to lower molecular weight
(say MW 4,00,000 to 10,00,000). Under more degradation, say when MW
falls to less than 1,50,000 NR gets changed to Liquid Rubber.
Presence of double bond
in NR also necessitates giving protection to degradation from the
attack of oxygen, ozone, etc., by way of incorporation of compounding
ingredients classified as Antidegradants.
Having described in
brief the salient points which contribute to the use of NR in rubber
products manufacture, let us try to trace out the development of the
synthetic analogue of NR namely the synthetic Cis 1, 4 Polyisprene.
Prior to 1954, all attempts to produce a replica or NR had ended up in
failures because the emulsion polymerisation technique being followed
then for the production of synthetic rubbers was not having precise
control on the geometry of the polymer molecule produced. Karl Zeigler
in Switzerland and Giulio Natta in Italy discovered during 1953/54 a
novel means for producing stereo regular polymers using special
catalysts and the dream of polymer chemists to synthesize NR came true
when in December 1954 Goodrich Company applied the Zeigler / Natta
catalysis for the discovery was that NR which was uniquely placed
before had to complete with its synthetic Cis Polyisoprene (IR) - one
having a Cis content of only 94% produced using Lithium and Lithium
Alkyls catalysts and a high Cis variety with about 98% Cis 1, 4 made
using Titanium Tetrachloride and Aluminium trialkyls as catalysts. In
the case of Ti-IR, the remaining 2% is 3, 4 structure and in Li-IR,
Cis 1, 4 is 90-92% trans 1, 4 is 2 - 3% and 3, 4 structure about 6 -
7%.
Let us now try to have
a comparative assessment of the different properties of NR and IR. It
is proposed to examine the raw rubber properties the processing
characteristics and technological properties of vulcanisates of NR and
IR. Conventional forms of NR like sheet and crepe are graded by means
of visual standards assessing the colour, presence or absence of
extraneous foreign matter (dirt), bubbles, blisters, etc., and within
each form there are different grades (35 grades in total). Visual
grading as it is subjective often leads to malpractices and trade
disputes and the criteria for grading often do not have any relation
to the technological properties of NR. In the case of IR, it is
specified by technical parameters which have some bearing on the
vulcanizate properties. In the latter half of 1960s, NR also began to
be made available in technically specified form akin to IR. Compact
bales of NR wrapped in dispersible thin polyethylene film facilitates
mechanical handling and protection from external contamination.
Colour of the raw
rubber is a very important factor for the selection for the
manufacture of certain items. A polymer with light colour is always
preferred in the production of light coloured rubber articles. A
comparison of this quality of NR and IR reveals that the conventional
grades of NR like smoked sheet and Estate Brown Crepe are dark
coloured and hence not the first choice whereas, IR is ideal. However,
Pale Latex Crepe (PLC) and light coloured crumb rubber from latex find
easy acceptance. Again purity of the polymer is the key factor guiding
selection of raw rubber for the manufacture of pharmaceutical rubber
goods. Here also IR is easily accepted.
GRADING
AND PACKING OF RUBBER